2. Overview
(a)The Fastest Ball Challenge
-Main theory/concept of the project:
Vectors and Resolving Vectors -
Introduction of the concept of vectors, and a useful tool, the scalar product, aka the dot product.
Vector quantity: Specified by a magnitude with apprrpriate units and has a direction. The convention for vectors in this manual is bold face, with an arrow on top of the quantity.
Useful properties of vectors:
Vector addition 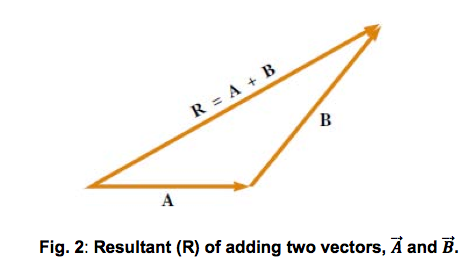
Vector subtraction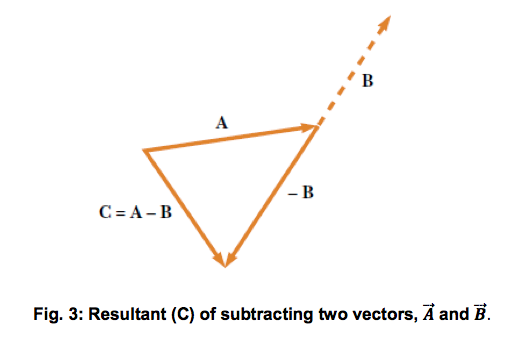
Since vectors have direction, a negative vector is just the same vector (positive) but pointing in the opposite direction (180° separation), as illustrated in Figure 3.
It is also helpful if we can decompose a vector into its component vectors, especially if we are using the Cartesian system (more commonly known when written as x, y, z).
Conservation of energy
3. Objectives
-To determine the angle of inclination for which the time of travel across the horizontal track will be least.
-Students will have to make use of the concepts of conservation of energy, kinetic and potential energy to win this challenge.
-Students will also make use of a stopwatch and photogate sensors in their data acquisition.
(b)Extraction of caffeine from beverages
-Main theory/concept of the project:
Solvent Extraction - A useful rule of thumb for judging solubility is “like dissolves like”. Organic compounds tend to be soluble in organic solvents. Polar materials, such as salts tend to be more soluble in polar solvents, such as water.
Some organic compounds are exceptions and are more soluble in water than in organic solvents. This is because they have a large number of polar functional groups, such as hydroxyl groups, relative to the amount of hydrocarbon. Glucose is an example of this kind of molecule.
In general, an organic compound and an inorganic salt can be easily separated using these solubility differences. If a mixture of 1,4-dimethoxybenzene and lithium chloride is dissolved in a mixture of diethyl ether and water, both will dissolve. T he mixture will separate into two clear layers. One will be the “organic layer” and it will contain the 1,4-dimethoxybenzene. T he other will be the aqueous layer, and it will contain the lithium chloride. If the organic layer is separated from the aqueous, dried to remove traces of water and then evaporated, pure 1,4-dimethoxybenzene will be obtained.
How can we tell which phase is aqueous and which is organic?
Hint: density. Think about your solutions and find out what your two layers are.
There are some other tricks that you need to know about. Usually, we do not extract once, but twice or even more times. This is to ensure that all traces of the phase are removed. Look out for this trick in the experimental procedure. Also, organic extracts must be dried after being separated from the aqueous layer. This is because organic solvents will contain small amounts of dissolved water. This is usually done by using a “drying agent” such as anhydrous magnesium sulfate or anhydrous sodium sulfate. These absorb the water from the organic solution.
Students often put a lot of effort into extractions by shaking the separatory funnel vigorously to mix the two layers. This can be a bad idea. Such vigorous mixing, can result in the formation of an emulsion – an intimate and inseparable mixture of the two layers. Emulsions can be broken – but not easily! Milk is an everyday example of an emulsion. Swirling the separatory funnel is a better technique.
Rotary Evaporator
This equipment is used to evaporate the solvent that your chemical compound of interest is solubilized in. It is composed of hot water bath (where you can adjust the temperature) and also a rotating glass tube (where you will connect your round bottom flask) connected in turn to a condenser and to vacuum. A teaching assistant will show you and explain the installation. Why do we rotate the flask while it is heating in water? Why do we need a condenser? And last but not least what is the benefit to work under vacuum (think about the relationship between pressure and temperature)?
No comments:
Post a Comment